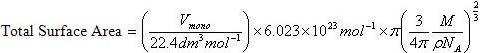What is Adsorption?
Adsorption is the phenomenon of accumulation of a large number of molecular species at the surface of a liquid or solid phase in comparison to the bulk. The term adsorption may be difficult to comprehend, and it's advisable to seek chemistry help online to better understand this concept.
How Adsorption occurs?
The process of adsorption arises due to presence of unbalanced or residual forces at the surface of liquid or solid phase. These unbalanced residual forces have tendency to attract and retain the molecular species with which it comes in contact with the surface. Adsorption is essentially a surface phenomenon.
Adsorption is a term which is completely different from Absorption .While absorption means uniform distribution of the substance throughout the bulk, adsorption essentially happens at the surface of the substance. When both Adsorption and Absorption processes take place simultaneously, the process is called sorption.
Adsorption process involves two components Adsorbent and Adsorbate. Adsorbent is the substance on the surface of which adsorption takes place.Adsorbate is the substance which is being adsorbed on the surface of adsorbent. Adsorbate gets adsorbed.
Adsorbate + Adsorbent gives rise to Adsorption

Oxygen molecules (red) adsorb on a bimetallic surface of platinum (purple) and cobalt (green). From P. B. Balbuena, D. Altomare, L. Agapito, and J. M. Seminario, “Theoretical analysis of oxygen adsorption on Pt-based clusters alloyed with Co, Ni, or Cr embedded in a Pt matrix,” J. Phys. Chem. B (in press).
Some modern techniques have been used to study surface.
- Low energy electron diffraction (LEED).
- Photo electron spectroscopy (PES).
- Scanning Tunneling microscopy (STM).
Adsorption in liquids
Adsorption can be understood by considering a simple example. In case of liquid state, water molecule present on the surface is attracted inwards by the molecules of water present in the bulk. This gives rise to surface tension. While the molecule of water present within the bulk is equally attracted from all the sides and the net force experienced by the water molecule in bulk is zero. This clearly shows that particles at surface and particles at the bulk are in different environment.
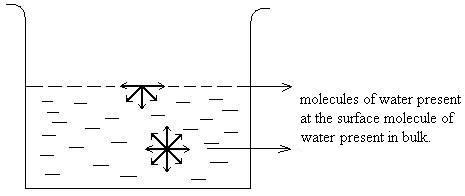
Water molecule on surface experiencing unbalanced forces as compared to molecule inside which experiences forces from all direction.
Adsorption in solids
In case of solid state these residual forces arises because a of unbalanced valence forces of atoms at the surface. The generation of these forces on solid surface can be explained diagrammatically as follows:
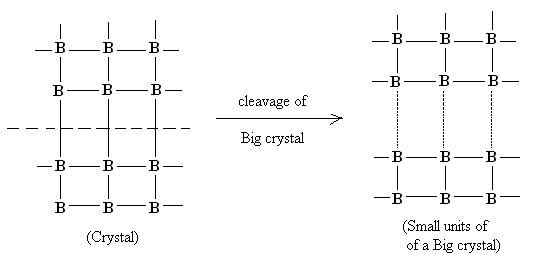
Cleavage of a big crystal into smaller unit
Due to cleavage of a big crystal into smaller unit, residual forces or vacancies gets generated on the surface of the solid. Occupancy of these vacancies by some other molecular species results into Adsorption.
Facts about Adsorption Process
Adsorption is a spontaneous process
For reaction or process to be spontaneous, there must be decreases in free energy of the system i.e. ΔG of the system must have negative value.
Also we know, ΔG = ΔH – TΔS
And during this process of adsorption, randomness of the molecule decreases which ΔS is negative. We can rewrite above equation as
![]()
Therefore for a reaction to be spontaneous ΔH has to be negative and
![]()
Adsorption is an exothermic process
Adsorption process takes place by adsorbate getting adsorbed on adsorbent .Forces of attraction exist between adsorbate and adsorbent and due to these forces of attraction, heat energy is released. So adsorption is an exothermic process.
Types of Adsorption
Forces of attraction exist between adsorbate and adsorbent. These forces of attraction can be due to Vanderwaal forces of attraction which are weak forces or due to chemical bond which are strong forces of attraction. On the basis of type of forces of attraction existing between adsorbate and adsorbent, adsorption can be classified into two types: Physical Adsorption or Chemical Adsorption.
Physical Adsorption or Physisorption
When the force of attraction existing between adsorbate and adsorbent are weak Vanderwaal forces of attraction, the process is called Physical Adsorption or Physisorption. Physical Adsorption takes place with formation of multilayer of adsorbate on adsorbent. It has low enthalpy of adsorption i.e. ΔHadsorption is 20-40KJ/mol.
It takes place at low temperature below boiling point of adsorbate.As the temperature increases in, process of Physisorption decreases.

Physical Adsorption vs. Temperature graph
Chemical Adsorption or Chemisorption
When the force of attraction existing between adsorbate and adsorbent are chemical forces of attraction or chemical bond, the process is called Chemical Adsorption or Chemisorption. Chemisorption takes place with formation of unilayer of adsorbate on adsorbent. It has high enthalpy of adsorption
i.e.
![]()
It can take place at all temperature. With the increases in temperature, Chemisorption first increases and then decreases.
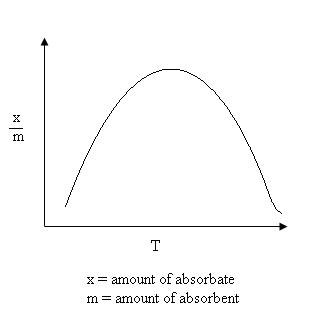
Chemical Adsorption vs. Temperature Graph
Adsorption Isotherm
The process of Adsorption is usually studied through graphs called as adsorption isotherm. It is the graph between the amounts of adsorbate (x) adsorbed on the surface of adsorbent (m) and pressure at constant temperature.
Applications of Adsorption
1. Charcoal is used as a decoloriser as it adsorbs the coloring matter from the coloured solution of sugar.

Pic Credit : Link
2. Silica gel adsorbs moisture from the desiccators.

Pic Credit : Link
3. Silica and alumina gels are used as adsorbents for removing moisture and for controlling humidity of rooms.
4. Activated charcoal is used in gas masks as it adsorbs all the toxic gases and vapours and purifies the air for breathing.

Pic Credit : Link
5 .Adsorption processes are useful in carrying out heterogeneous catalysis.
Factors on which Adsorption Depends
Temperature
Adsorption increases at low temperature conditions.
![]()
Adsorption process is exothermic in nature. According to Le Chatleir principle, low temperature conditions would favour the forward direction.
Pressure
As depicted by Adsorption Isotherm, with the increases in pressure, adsorption increases up to a certain extent till saturation level is achieved. After saturation level is achieved no more adsorption takes place no matter how high the pressure is applied.
Surface Area
Adsorption is a surface phenomenon therefore it increases with increase in surface area.
Activation of Adsorbent
Activation of adsorbent surface is done so as to provide more number of vacant sites on surface of adsorbent. This can be done by breaking solid crystal in small pieces, heating charcoal at high temperature, breaking lump of solid into powder or other methods suitable for particular adsorbent.
Surface Area of Adsorbent
As adsorption is a surface phenomenon, surface area offered by Adsorbent becomes important factor for consideration.
Volume of an ideal gas at STP = 22.4 L= 22.4 dm³
Also the number of gaseous molecules present at STP = 6.023*10²³ molecules
Vmono be the adsorbed volume of gas at high pressure conditions so as to cover the surface with a unilayer of gaseous molecules. Let the total number of molecules of gas adsorbed corresponding to volume Vmono be N whose value is given as
![]()
Now area of a molecule having density ρ and occupying volume V is calculated as follows
![]()
Where Vm is the total volume of the surface
Now Total volume Vm is equal to volume of each molecule (V) multiplied by total number of molecule (NA) adsorbed on the surface at volume, V.
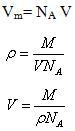
Molecule being spherical in nature, Volume of the molecule V is also given as
![]()
The radius, r occupied by each gas molecule is
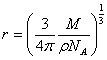
Area occupied by each gas molecule is
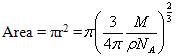
This is the area occupied by one molecule. If the value multiplied by the total number of molecules adsorbed on the surface of adsorbent we will get the total surface area of the adsorbent.
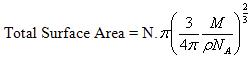
Or,