What is Langmuir Adsorption Isotherm?
In 1916, Irving Langmuir proposed another Adsorption Isotherm which explained the variation of Adsorption with pressure. Based on his theory, he derived Langmuir Equation which depicted a relationship between the number of active sites of the surface undergoing adsorption and pressure.
Assumptions of Langmuir Isotherm
Langmuir proposed his theory by making following assumptions.
1. Fixed number of vacant or adsorption sites are available on the surface of solid.
2. All the vacant sites are of equal size and shape on the surface of adsorbent.
3. Each site can hold maximum of one gaseous molecule and a constant amount of heat energy is released during this process.
4. Dynamic equilibrium exists between adsorbed gaseous molecules and the free gaseous molecules.

Where A (g) is unadsorbed gaseous molecule, B(s) is unoccupied metal surface and AB is Adsorbed gaseous molecule.
5. Adsorption is monolayer or unilayer.
Derivations of the Langmuir Adsorption Equation
Calculation of Equilibrium Constant
Langmuir proposed that dynamic equilibrium exists between adsorbed gaseous molecules and the free gaseous molecules. Using the equilibrium equation, equilibrium constant can be calculated.
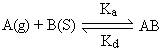
Where Ka represents equilibrium constant for forward reaction and Kd represents equilibrium constant for backward direction.
According to Kinetic theory,
Rate of forward reaction = Ka [A] [B]
Rate of backward reaction = Kd [AB]
At equilibrium, Rate of forward reaction is equal to Rate of backward reaction

The above equation represents the equilibrium constant for distribution of adsorbate between the surface and the gas phase.
Derivation
Langmuir Equation which depicts a relationship between the number of active sites of the surface undergoing adsorption (i.e. extent of adsorption) and pressure.
To derive Langmuir Equation and new parameter ‘ θ ’ is introduced. Let θ the number of sites of the surface which are covered with gaseous molecules. Therefore, the fraction of surface which are unoccupied by gaseous molecules will be (1 – θ).
Now, Rate of forward direction depends upon two factors: Number of sited available on the surface of adsorbent, (1 – θ) and Pressure, P. Therefore rate of forward reaction is directly proportional to both mentioned factors.

Similarly, Rate of backward reaction or Rate of Desorption depends upon number of sites occupied by the gaseous molecules on the surface of adsorbent.
![]()
At equilibrium, rate of adsorption is equal to rate of desorption.
Ka P (1 – θ) = Kd θ
We can solve the above equation to write it in terms of θ.
KaP – KaP θ = Kd θ
KaP = KaP θ + Kd θ
KaP = (Kd + KaP) θ
![]()
Divide numerator and denominator on RHS by Kd, we get

Now put
![]()
in above equation we get
![]()
Langmuir Adsorption Equation
This is known as Langmuir Adsorption Equation.
Alternate form of Langmuir Adsorption Equation
Langmuir adsorption equation can be written in an alternate form in terms of volume of gas adsorbed. Let V be volume of gas adsorbed under given sets of conditions of temperature and pressure and Vmono be the adsorbed volume of gas at high pressure conditions so as to cover the surface with a unilayer of gaseous molecules.
![]()
Substituting the value of θ in Langmuir equation

Or in terms of pressure P we get,
![]()
Langmuir Adsorption Equation in alternate form
Thus, if we plot a graph between P/V Vs P, we will obtain a straight line with
![]()
Limitations of Langmuir Adsorption Equation
- The adsorbed gas has to behave ideally in the vapor phase. This condition can be fulfilled at low pressure conditions only. Thus Langmuir Equation is valid under low pressure only.
- Langmuir Equation assumes that adsorption is monolayer. But, monolayer formation is possible only under low pressure condition. Under high pressure condition the assumption breaks down as gas molecules attract more and more molecules towards each other. BET theory proposed by Brunauer, Emmett and Teller explained more realistic multilayer adsorption process.
- Another assumption was that all the sites on the solid surface are equal in size and shape and have equal affinity for adsorbate molecules i.e. the surface of solid if homogeneous. But we all know that in real solid surfaces are heterogeneous.
- Langmuir Equation assumed that molecules do not interact with each other. This is impossible as weak force of attraction exists even between molecules of same type.
- The adsorbed molecules has to be localized i.e. decrease in randomness is zero (ΔS = 0).This is not possible because on adsorption liquefaction of gases taking place, which results into decrease in randomness but the value is not zero.
From above facts we can conclude that, Langmuir equation is valid under low pressure conditions.
Freundlich Adsorption Equation: A Special Case of Langmuir Equation
We consider Langmuir Equation
![]()
At low pressure value of KP<<1. Therefore,
![]()
The above equation shows linear variation between extent of adsorption of gas and pressure.
At high pressure value of KP>>1
![]()
The extent of adsorption, θ is independent of pressure at high pressure conditions. The reaction at this stage becomes zero order
Combining the results of equation (4) and (5), we can conclude that
![]()
Equation (3) is in agreement with Freundlich adsorption equation.
We can say that Freundlich adsorption equation is a special case of Langmuir equation.
