 This article has been authored by Geetika Arora, a qualified Chemistry educator.With her write-ups,she tends to make every complex Chemistry concept look dead simple to perplexed minds. She has exhaustively written Chemistry related articles at XAmplified, explaining various concepts and phenomenons.
This article has been authored by Geetika Arora, a qualified Chemistry educator.With her write-ups,she tends to make every complex Chemistry concept look dead simple to perplexed minds. She has exhaustively written Chemistry related articles at XAmplified, explaining various concepts and phenomenons.
Table Of Content
What is rubber?
Rubber is a natural polymer of Isoprene (2-Methyl -1, 3 – Butadiene). It is a linear, 1, 4 – addition polymer of Isoprene.
Natural rubber has elastic properties and it undergoes long range reversible extension even if relatively small force is applied to it. Therefore, it is also known as Elastomer. Natural rubber is prepared from latex which is a Colloidal Solution of Rubber in Water.
Where is Rubber found?
Rubber trees are basically found in tropical & semitropical countries. Indonesia, Malaysia, Sri Lanka, South America and India (especially Kerala, Tamilnadu and Karnataka) have abundant resource of natural rubber.

Rubber Plantation in Kerala, India
Structure of Natural Rubber
Natural Rubber is a polymer of Isoprene. To understand the structure of Rubber we shall concentrate on structure of Isoprene. Isoprene is a conjugated diene containing double bonds at alternate position.
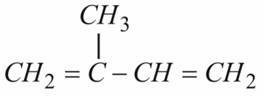
Structure of Isoprene: Monomer of Natural Rubber
Isoprene undergoes free radical polymerization like substituted ethylene. Isoprene polymerizes to give Polyisoprene polymer, a simple alkene having each unit still containing one double bond.
Now, polymerization of Isoprene may follow either of the two pathways; either of cis-polymerization or trans-polymerization. The rubber formed from cis-polymerization is called cis-Polyisoprene or Natural Rubber. Similarly, the rubber formed from trans-polymerization is called Synthetic Rubber.

Isoprene (2-Methyl -1, 3-butadiene) undergoes cis-polymerization to form natural rubber

Structure of Natural Rubber (Cis Polyisoprene)
By observing structure of natural rubber we can infer that there is no polar group in this structure. As a result of this the intermolecular forces of attraction are weak Vanderwaal forces of attraction. These forces of attraction are further weakened because of the cis-configuration of all the double bonds that does not permit the close interaction of polymer chains. Thus Natural Rubber (Cis Polyisoprene) does not have a straight chain but has a coiled structure. As a result of this, it gets elastic property.
Synthetic Rubber
Polymerization of deines (molecules containing double bond) to form substitutes for rubber is the forerunner of the enormous present day plastic industry. Chloroprene was the first commercially successful rubber substitute produced in the United States.
![]()
Chloroprene undergoing trans-polymerization to produce Synthetic Rubber, Polychloroprene
The properties of Rubber so formed are determined by the nature of the substitutent groups. For example, Polychloroprene is inferior to natural rubber in some properties but superior in its resistance to oil, Organic Solvents. These differences are due to difference in nature of their monomers: Isoprene (for natural rubber) and Chloroprene (for synthetic rubber).
Synthetic Rubber (also known as Gutta-Percha) was obtained by the free radical polymerization of Isoprene. The rubber so formed has all trans- Configuration. As a result of this, synthetic rubber has a highly regular zig-zag chain which cannot be stretched .This accounts for non-elasticity of Synthetic Rubber.
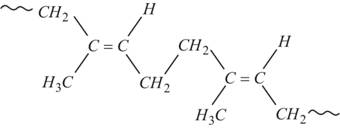
Structure of Synthetic Rubber (trans – Polyisoprene, Gutta-percha)
Types of Synthetic Rubber
Neoprene
Neoprene is a polymer of chloroprene. It is also known as Polychloroprene. To synthesize Neoprene its monomer Chloroprene is required. Chloroprene required for this process is synthesized from Vinylacetylene which performs Markonikov addition under acidic condition to produce Chloroprene.
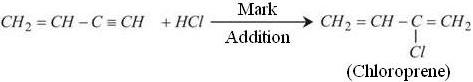
The Vinylacetylene required for above reaction is prepared by Dimerization of acetylene by passing it through an aqueous solution of Ammonium Chloride and Cuprous Chloride at 343K.

The Chloroprene obtained undergoes Polymerization to gives Neoprene. Though no specific catalysts are needed for this process but the polymerization becomes faster in the presence of Oxygen or peroxide.
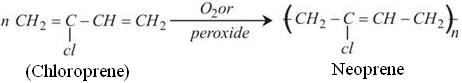
Note: Polymerization of Chloroprene is 700 times faster than Isoprene.
Uses of Neoprene
It is used in the manufacture of hoses, gaskets, shoe heels, stoppers, conveyor belts and printing rollers etc. It is also used as an insulator.
BUNA–S
BUNA–S is a copolymer of a mixture of 1,3- Butadiene and styrene in the ratio of 3:1 in the presence of sodium (which is polymerizing agent) gives styrene – butadiene copolymer (styrene – butadiene rubber) or BUNA –S. The name BUNA–S is made up of Bu which indicates 1, 3 – Butadiene, NA is for Sodium (Na) and S indicates Styrene.

Properties of Buna–S
- It is very tough and a good substitute for natural rubber.
- It possesses high abrasion resistance.
- It has high load bearing capacity.
Uses of Buna–S
- It is used for manufacturing automobile tyres.
- It is used for making floor tiles, footwear components, cable insulation etc.
BUNA – N (Nitrile Rubber)
BUNA–N is obtained by copolymerization of 1, 3 – Butadiene and acrylonitrile in presence of a peroxide catalyst. The name BUNA–N is made up of Bu which indicates 1, 3 – Butadiene, NA is for Sodium (Na) and N indicates acrylonitrile.

1, 3-Butadiene reacts with Acrylonitrile to produce Buna – N
Properties of BUNA–N
BUNA-N is resistant to the action of petrol, lubricating oils and organic solvents.
Uses of BUNA–N
BUNA-N It is used in making oil seals, hoses, tank linings etc.
Thiokol
Thiokol is prepared by copolymerization of 1, 2 – dichloroethane (ethylene dichloride) with Sodium Tetrasulphide (Na2S4) in presence of Magnesium hydroxide.
Properties of Thiokol
Thiokol is resistant to the action of mineral oils, solvents, oxygen & ozone.
Note : Thiokol is also known as polysulphide rubber. It’s tensile strength is slightly less than that of Natural rubber. It’s different from vulcanized Rubber.
Vulcanized Rubber
Natural rubber is not an important polymer for commercial purpose because of its softness & tacky (sticky) properties. Softness of natural rubber increases with the increase in temperature while brittleness increases at low temperature.
Therefore, ideal temperature for using Rubber is 283 – 335K where its elasticity is maintained. Other properties which decrease the quality of natural rubber are:
- It has large water absorption capacity
- It has low tensile strength & low resistance to abrasion
- It is not – resistant to abrasion
- It is easily attacked by organic reagents.
The properties of Natural rubber can be improved by a process called Vulcanization .Vulcanization is the process of introduction of Sulphur bridges between different chains by heating raw rubber with Sulphur at 373-415K. In the absence of catalyst the process of vulcanization is slow. Some additive such as Zinc Oxide is added to accelerate the rate of Vulcanization.
Difference between Vulcanized rubber and Natural rubber
The new or vulcanized rubber obtained has properties that are just opposite to that of natural rubber. These properties are
- Vulcanized rubber has excellent elasticity.
- Low water absorption tendency
- It is resistant to the action of organic solvents
- It is resistant to attack of oxidizing agents.
Vulcanized Rubber is an improved form of Natural rubber.
In vulcanized rubber, Sulphur bridges are introduced either at their reactive allylic sites or at the site of double bond. The presence of double bond in the rubber molecule makes it’s highly reactive as it provides allylic hydrogen that permits formation of Cross links between different chains. The presence of these cross links increases the toughness, strength and hardness of rubber. Due to the presence of Sulphur bridges, individual chains can no longer slip over one another but are locked together in a giant size molecule.
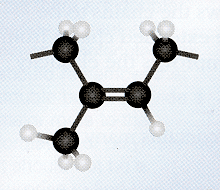
Cross-links being formed between the rubber polymer chains during Vulcanization
Note : Amount of Sulphur used for vulcanization process determines the extent of hardness or toughness of the rubber.5% Sulphur is used for making tyre rubber, 20 – 25% Sulphur is used for making Ebonite. 30% of Sulphur is for making Battery Case Rubber.
Fact : The process of Vulcanization was discovered by Charles good year in 1839.
References
- Robert T. Morrison, Robert N. Boyd, Organic Chemistry, 6th Edition, Publisher Prentice Hall (January 17, 1992)
- Rubber Plantation, link : farm1.static.flickr.com/149/406843133_0ac7ce1cff.jpg?v=0
- BBC/OU Open2.net – The World Around Us – Lost at sea – Explore the science – Rubber & vulcanization, link : open2.net/sciencetechnologynature/worldaroundus/explore-rubber_and_vulcanisation.html
- RLG Car Tyres (Custom image), link : rlgtyres.co.uk/car/rlg_tyres_car/rlg_car_tyres_eagle_f1.jpg
